







ACE-031 1mg
From CAD $113 CAD $125
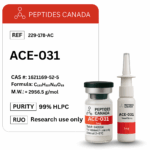
Contents: ACE-031 (ActRIIB–Fc Fusion Protein)
Form: Powder
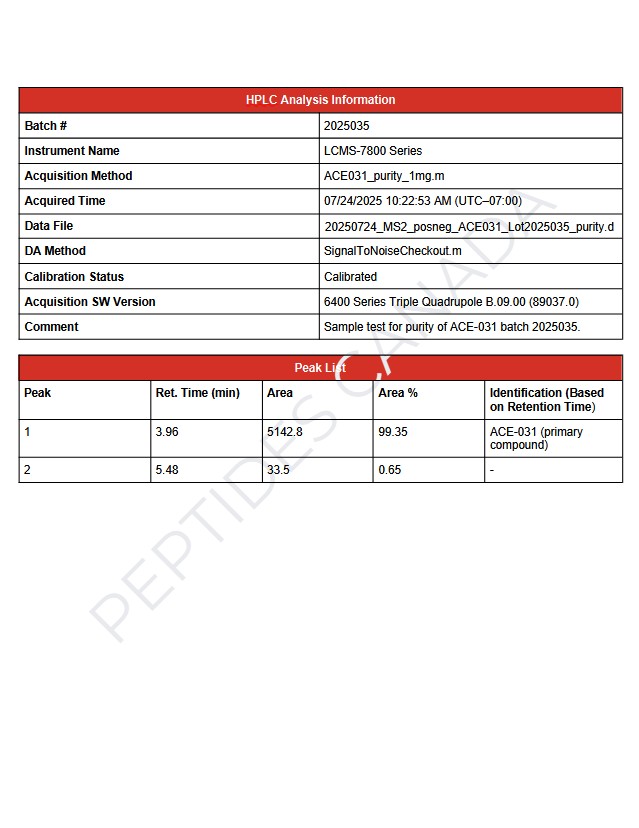
Purity: 99.3%
TESTED FOR:
- PURITY
- STERILITY
- WEIGHT
- ENDOTOXINS(LPS)

Free Reconstitution solution automatically added to your cart with each order of vial.
This product is Made, Tested & Shipped From Canada.
Ships Today
Order by 1:00 PM EST
Free Shipping
For 2 or more vials

99%+ Purity Guaranteed

COA Verified+
Trackable Shipping
ACE-031 (Activin Type IIB Receptor-Fc Fusion Protein)
ACE-031 is a recombinant fusion protein composed of the extracellular domain of the human activin receptor type IIB (ActRIIB) linked to the Fc domain of human IgG1. It acts as a soluble decoy receptor that binds to activins and myostatin (GDF-8), thereby inhibiting their signaling through endogenous ActRIIB receptors. This mechanism increases muascle mass and strength in preclinical models by reducing negative regulation of skeletal muscle growth.
Overview
ACE-031 was designed to block signaling from members of the transforming growth factor-β (TGF-β) superfamily, primarily myostatin and activins, which suppress muscle growth and differentiation. By sequestering these ligands, ACE-031 prevents their interaction with cell-surface receptors, leading to enhanced myogenesis and hypertrophy in muscle tissue.
Research applications focus on muscle wasting, sarcopenia, cachexia, and neuromuscular disorders such as Duchenne muscular dystrophy (DMD). The molecule serves as a critical research tool in studying the ActRIIB–myostatin pathway and anabolic regulation in skeletal muscle.
Chemical Makeup
- Type: Recombinant human fusion protein
- Structure: Soluble extracellular domain of ActRIIB linked to human IgG1 Fc
- Molecular Weight: ~95–100 kDa (monomeric)
- Expression System: Recombinant CHO cells
- Purity: ≥98% (per COA)
- Form: Lyophilized protein
- Size: 1 mg, 2 mg, and 5 mg vials available
Research and Clinical Studies
Myostatin and Activin Inhibition
ACE-031 binds circulating myostatin and activins, neutralizing their inhibitory effects on skeletal muscle growth. Preclinical models demonstrate increased muscle fiber diameter, lean mass, and grip strength following administration.
Muscle and Bone Metabolism Research
In addition to muscle effects, ACE-031 has been shown to increase bone mineral density and improve markers of bone formation, suggesting cross-talk between muscle and bone anabolic pathways.
Neuromuscular Disease Models
ACE-031 has been evaluated in mouse and primate models of Duchenne muscular dystrophy (DMD), where it improved muscle mass and functional outcomes, supporting its use in studies of muscle degenerative disease mechanisms.
Metabolic and Energy Balance
By increasing lean body mass and reducing adiposity, ACE-031 serves as a model compound in research investigating muscle metabolism, insulin sensitivity, and energy expenditure.
Pharmacokinetics
ACE-031 exhibits an extended circulating half-life due to the Fc fusion, allowing for intermittent dosing in experimental protocols (typically 3–4 days in rodents; longer in primates).
ACE-031 is available for research and laboratory purposes only. Not for human consumption.
References
- Lee SJ, et al. Regulation of muscle mass by myostatin and activin signaling. Nat Rev Mol Cell Biol. 2020;21(5):269–280. https://pubmed.ncbi.nlm.nih.gov/32152592/
- Lach-Trifilieff E, et al. Inhibition of ActRIIB signaling increases muscle mass and strength. Proc Natl Acad Sci U S A. 2014;111(17):E1774–E1782. https://pubmed.ncbi.nlm.nih.gov/24706799/
- Cadena SM, et al. Administration of ACE-031 increases muscle mass and reduces fat in preclinical models. Am J Physiol Endocrinol Metab. 2010;299(6):E965–E974. https://pubmed.ncbi.nlm.nih.gov/20858706/
- Campbell C, et al. Pharmacologic blockade of myostatin in Duchenne muscular dystrophy: results of a clinical trial with ACE-031. Muscle Nerve. 2017;55(4):458–464. https://pubmed.ncbi.nlm.nih.gov/27560668/
- Lawlor MW, et al. Inhibition of myostatin signaling improves muscle function in DMD models. Skelet Muscle. 2011;1(1):34. https://pubmed.ncbi.nlm.nih.gov/22087763/
- Rodino-Klapac LR, et al. Gene therapy and myostatin blockade in muscle disorders. Mol Ther. 2013;21(1):132–140. https://pubmed.ncbi.nlm.nih.gov/23151419/
- Ploquin C, et al. ActRIIB blockade alters muscle and bone composition in primates. J Endocrinol. 2012;213(2):183–195. https://pubmed.ncbi.nlm.nih.gov/22495698/
- Morine KJ, et al. Systemic inhibition of activin type II receptors promotes muscle growth and fat loss. Am J Physiol Endocrinol Metab. 2010;299(5):E776–E788. https://pubmed.ncbi.nlm.nih.gov/20858707/
- Attie KM, et al. Clinical pharmacology of ACE-031 in healthy volunteers. Muscle Nerve. 2013;47(3):416–423. https://pubmed.ncbi.nlm.nih.gov/23335387/
- Campbell C, et al. Safety and pharmacodynamic effects of ACE-031 in DMD boys: phase 1 study. Neurology. 2012;78(8):627–635. https://pubmed.ncbi.nlm.nih.gov/22357796/

HIGHEST QUALITY PEPTIDES
Our products are scientifically formulated and manufactured in cGMP-compliant facilities.

FAST DELIVERY
Enjoy fast and reliable 3–5 day shipping.

Dedicated Customer Service
Our customer service team is highly knowledgeable in peptide research and its applications. We’re available 24/7 to assist you.

Tested. Verified. Trusted.
We take a laboratory-first approach to quality. Each batch is made under controlled conditions and verified by an independent lab (HPLC/MS). We only ship batches that test ≥99% purity, and we provide a full COA, including identity, methods, and chromatograms, for your review.
See the Process for Yourself
We make our peptides in our own cGMP lab. Watch the video to see how every vial is produced, tested, and handled with care.
Science Behind Our Peptides
A clear explanation of how our peptides work, their benefits, why quality matters for best results, and what you should know.
Categories
Categories
How do I know the peptides I order are exactly what the label says?
Every vial we sell comes from a lab that follows current Good Manufacturing Practices (cGMP). That means each step of production is documented and controlled. Before a batch is released, it’s tested by independent third-party labs for purity, identity, and sterility. Certificates of analysis are available so you can see the exact test results.
Are your peptides produced in a sterile and controlled environment?
Yes. The labs we work with use ISO-certified clean rooms where air quality, equipment, and handling procedures are tightly regulated. Staff are trained to pharmaceutical-grade standards. This ensures the peptides are produced in an environment that minimizes contamination risks.
What about shipping? Do the peptides remain stable in transit?
Peptides in lyophilized (freeze-dried) form are stable at room temperature for transport. Once you receive them, refrigeration is recommended to maintain long-term integrity. We package every order securely to prevent damage and ship promptly, so your vials arrive in optimal condition.
How do I know you actually make these peptides yourselves?
We operate under strict in-house protocols that follow current Good Manufacturing Practices (cGMP). That means our team oversees the entire process from sourcing raw amino acids to the final lyophilized vial. Nothing is outsourced or repackaged. This gives us full control over purity, consistency, and sterility, and it’s why we can stand behind every single vial we ship.
What should I do with the vials once they arrive?
Store them in the refrigerator, away from direct light and heat. If you need to keep them longer, some peptides can be stored frozen. Each vial comes with clear handling instructions so you know the proper conditions for stability.
What proof do you have that your peptides are legitimate?
The strongest proof is transparency. For every peptide, we can provide certificates of analysis, manufacturing documentation, and references to the published scientific research behind it. If you ever have questions, we’ll show you the data rather than ask you to take our word for it.