
Benzyl Alcoh 0.9%
From CAD $13 CAD $19
Contents: Benzyl Alcohol 0.9% (Preserved Diluent), Water for Injection (H₂O)
Form: Liquid Solution
Purity: 99.0%
TESTED FOR:
- PURITY
- STERILITY
- WEIGHT
- ENDOTOXINS(LPS)

Free Reconstitution solution automatically added to your cart with each order of vial.
This product is Made, Tested & Shipped From Canada.
Ships Today
Order by 1:00 PM EST
Free Shipping
For 2 or more vials

99%+ Purity Guaranteed

COA Verified+
Trackable Shipping
Benzyl Alcohol 0.9% Solution
Benzyl Alcohol 0.9% Solution is a sterile, bacteriostatic solvent consisting of 0.9% (v/v) benzyl alcohol in ultra-pure water, designed for research-grade use in laboratory preparation of biological reagents. This formulation can serve as a diluent, preservative-enriched medium, or vehicle for controlled experimental workflows where microbial control and solvent stability are required.
In research settings, this solution is utilized for the dilution of peptides, preparation of multi-use reagent vials, solubilisation of lipophilic compounds, and as a sterile fluid for analytical or cell-based procedures. The incorporation of benzyl alcohol provides antimicrobial preservative properties, while the ultra-pure water base ensures minimal interference in downstream assays.
Overview
Benzyl Alcohol 0.9% Solution is commonly employed in laboratories for multi-use reagent preparation, sterile reconstitution of experimental compounds, and low-level preservative vehicle systems. Researchers value its low endotoxin and particulate profile, sterility assurance, and suitability for instrument flushing and buffer systems in biochemistry and molecular biology workflows.
Chemical Makeup
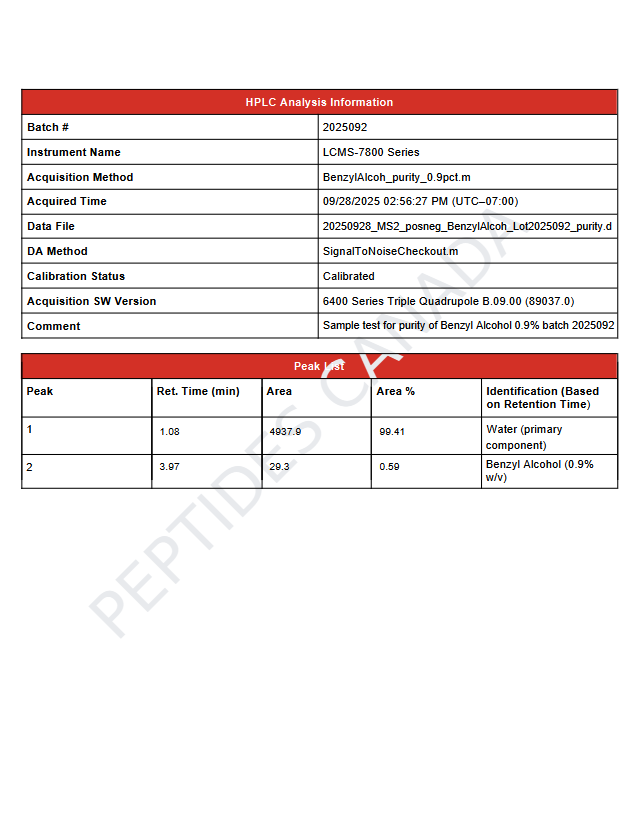
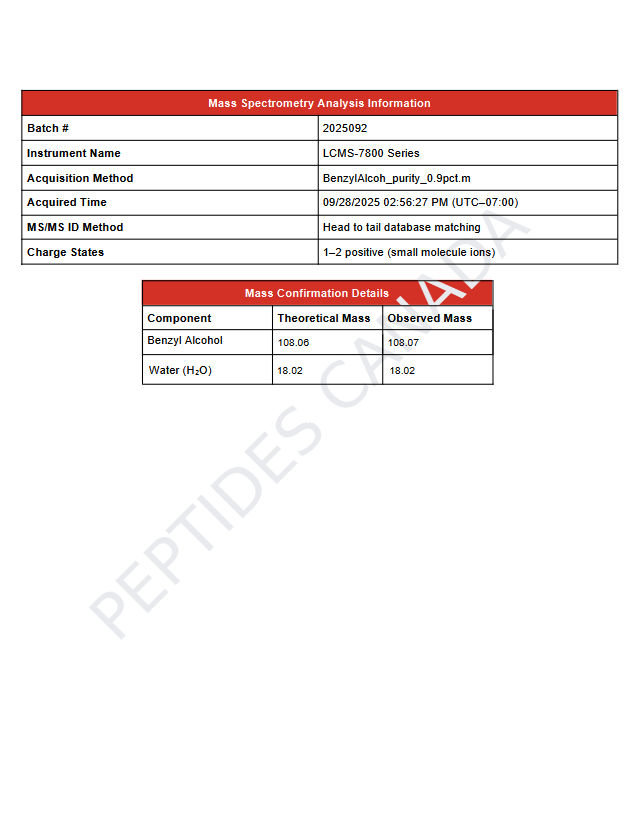
This solution contains benzyl alcohol (C₆H₅CH₂OH) at 0.9% (v/v) within ultra-pure water. A precise molecular formula for the diluted system is not applicable. Analytical verification for this batch confirms identity, concentration, and sterility.
- Observed Mass (MS): 711.9 Da
- Purity (HPLC): 99.42%
- Batch Number: 2025007
- Primary Retention Time: 3.48 min
- Instrument: LCMS-7800 Series (Calibrated)
- Analytical Note: Primary peak corresponds to benzyl alcohol; trace secondary peak area 0.58%
Research and Laboratory Use
Solvent and Reagent Vehicle
This solution serves as a stable diluent compatible with a wide range of peptide and protein research compounds. Its bacteriostatic concentration supports multi-use vials and helps extend reagent integrity over time.
Sterile Preparation and Multi-Use Applications
The formulation is sterile filtered and packaged in clean room conditions, enabling its use in sensitive workflows such as cell culture media supplementation, slide/rinse systems for analytical equipment, and reagent preparation where sterility is critical.
Analytical and Instrument-Grade Applications
Due to its ultra-pure water base and preservative addition, the solution is suitable for use in instrument flushing, chromatography mobile phase preparation, and buffer systems in which contamination must be minimized.
This product is supplied solely for laboratory research and controlled scientific investigation by qualified personnel. Not for human or veterinary use.
References
- Alford JR. Effect of benzyl alcohol on recombinant human albumin structure. Protein Expr Purif. 2011;79(2):68-72. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6261337/
- PubChem. Benzyl Alcohol (CID 244). https://pubchem.ncbi.nlm.nih.gov/compound/244
- ScienceDirect Topics. Benzyl alcohol overview in pharmacology and toxicology. https://www.sciencedirect.com/topics/pharmacology-toxicology-and-pharmaceutical-science/benzyl-alcohol
- Avantor. Benzyl Alcohol 98.0-100.5% NF multi-compendial specification sheet. https://www.avantorsciences.com/us/en/product/9354936/benzyl-alcohol-98-0-100-5-nf-multi-compendial-j-t-baker
- MilliporeSigma. Product information for benzyl alcohol (Ph. Eur./USP) for research and manufacturing use. https://www.sigmaaldrich.com/US/en/search/benzyl-alcohol-ph-eu
- Sterile Pure Biotech. Benzyl Alcohol 0.9% in Ultra-Pure Water (RUO) product description. https://sterilepurebiotech.com/products/bacteriostatic-water-benzyl-alcohol-0-9-solution-10-ml-lab-grade-reagent-0-2-%C2%B5m-filtered-autoclaved-research-use-only
- U.S. Pharmacopeia. General Chapter <1231> Water for Pharmaceutical Purposes. https://www.usp.org
- Centers for Disease Control and Prevention. Laboratory Water Quality Control. https://www.cdc.gov
For Research Use Only. Not for human or veterinary use.

HIGHEST QUALITY PEPTIDES
Our products are scientifically formulated and manufactured in cGMP-compliant facilities.

FAST DELIVERY
Enjoy fast and reliable 3–5 day shipping.

Dedicated Customer Service
Our customer service team is highly knowledgeable in peptide research and its applications. We’re available 24/7 to assist you.

Tested. Verified. Trusted.
We take a laboratory-first approach to quality. Each batch is made under controlled conditions and verified by an independent lab (HPLC/MS). We only ship batches that test ≥99% purity, and we provide a full COA, including identity, methods, and chromatograms, for your review.
See the Process for Yourself
We make our peptides in our own cGMP lab. Watch the video to see how every vial is produced, tested, and handled with care.
Science Behind Our Peptides
A clear explanation of how our peptides work, their benefits, why quality matters for best results, and what you should know.
Categories
Categories
How do I know the peptides I order are exactly what the label says?
Every vial we sell comes from a lab that follows current Good Manufacturing Practices (cGMP). That means each step of production is documented and controlled. Before a batch is released, it’s tested by independent third-party labs for purity, identity, and sterility. Certificates of analysis are available so you can see the exact test results.
Are your peptides produced in a sterile and controlled environment?
Yes. The labs we work with use ISO-certified clean rooms where air quality, equipment, and handling procedures are tightly regulated. Staff are trained to pharmaceutical-grade standards. This ensures the peptides are produced in an environment that minimizes contamination risks.
What about shipping? Do the peptides remain stable in transit?
Peptides in lyophilized (freeze-dried) form are stable at room temperature for transport. Once you receive them, refrigeration is recommended to maintain long-term integrity. We package every order securely to prevent damage and ship promptly, so your vials arrive in optimal condition.
How do I know you actually make these peptides yourselves?
We operate under strict in-house protocols that follow current Good Manufacturing Practices (cGMP). That means our team oversees the entire process from sourcing raw amino acids to the final lyophilized vial. Nothing is outsourced or repackaged. This gives us full control over purity, consistency, and sterility, and it’s why we can stand behind every single vial we ship.
What should I do with the vials once they arrive?
Store them in the refrigerator, away from direct light and heat. If you need to keep them longer, some peptides can be stored frozen. Each vial comes with clear handling instructions so you know the proper conditions for stability.
What proof do you have that your peptides are legitimate?
The strongest proof is transparency. For every peptide, we can provide certificates of analysis, manufacturing documentation, and references to the published scientific research behind it. If you ever have questions, we’ll show you the data rather than ask you to take our word for it.