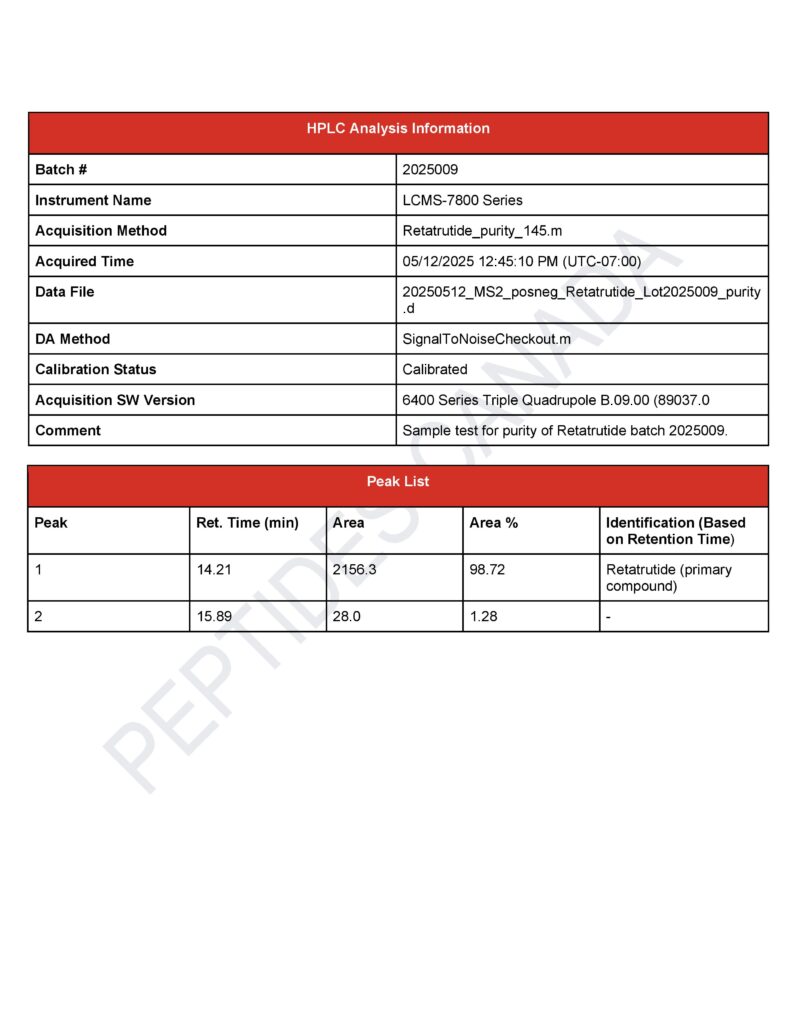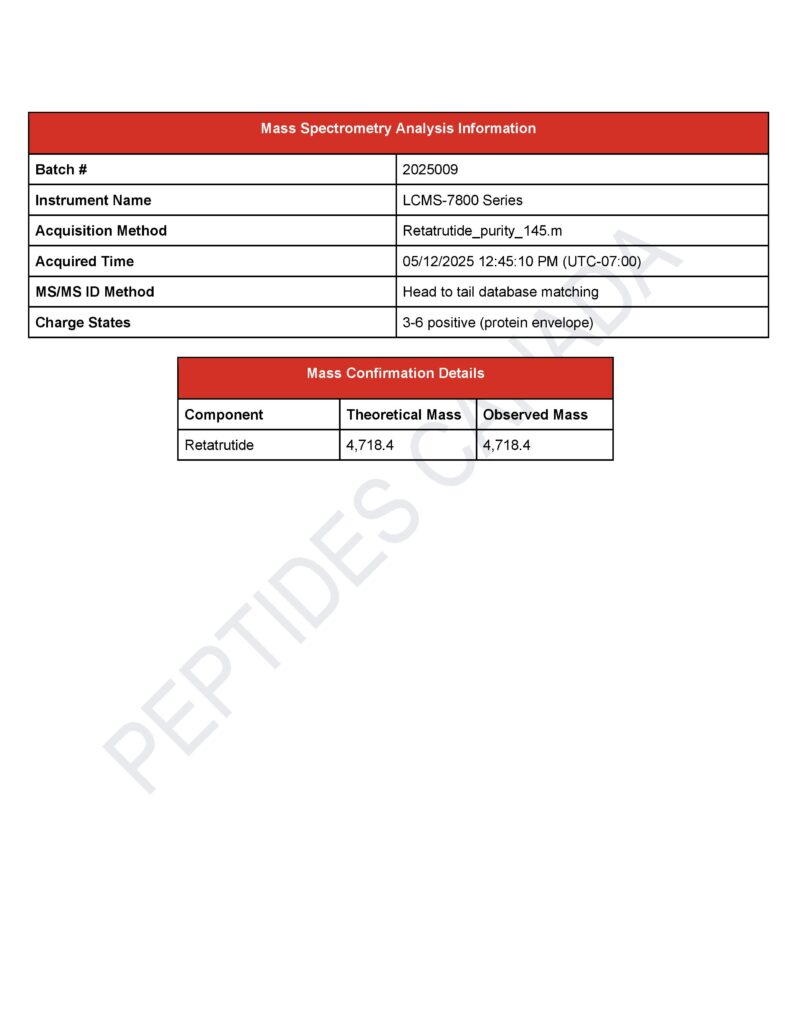
Retatrutide Triple Agonist
From CAD $90 CAD $300
Contents: Retatrutide
Form: Powder
Purity: 99.1%
TESTED FOR:
- PURITY
- STERILITY
- WEIGHT
- ENDOTOXINS(LPS)

Free Reconstitution solution automatically added to your cart with each order of vial.
This product is Made, Tested & Shipped From Canada.
Ships Today
Order by 1:00 PM EST
Free Shipping
For 2 or more vials

99%+ Purity Guaranteed

COA Verified+
Trackable Shipping
Retatrutide Peptide
Retatrutide is a synthetic polypeptide that functions as a next-generation triple incretin receptor agonist. It targets three key metabolic hormone receptors – GLP-1 (glucagon-like peptide-1), GIP (glucose-dependent insulinotropic polypeptide), and glucagon – concurrently. By activating these pathways, retatrutide is being investigated for its potential to holistically manage metabolic disorders, including obesity, type 2 diabetes, and non-alcoholic fatty liver disease (NAFLD). This multi-receptor approach may allow retatrutide to modulate appetite, glucose homeostasis, and energy expenditure simultaneously, distinguishing it from single- or dual-agonist therapies.
Overview
Researchers note that retatrutide exerts its effects via agonism of three key hormone receptors:
GLP-1 Receptors: Activation may enhance insulin secretion, slow gastric emptying, and increase satiety, helping to lower blood glucose and reduce food intake.
GIP Receptors: Agonism can further stimulate insulin release and influence fat metabolism, potentially working synergistically with GLP-1 pathways to improve glycemic control.
Glucagon Receptors: Stimulation increases energy expenditure and promotes fatty acid oxidation, which may lead to reduced body fat and improvement in liver fat content.
By concurrently targeting these incretin and glucagon receptors, retatrutide is designed to provide comprehensive metabolic regulation. Studies suggest that engaging all three pathways yields synergistic effects on weight reduction and glycemic control beyond those of single-hormone therapies. Preclinical research has shown, for example, that retatrutide delays gastric emptying and reduces caloric intake, contributing to greater weight loss compared to GLP-1 agonists alone. The addition of glucagon receptor activity is also thought to boost metabolic rate and enhance fat utilization – notably in the liver – providing benefits such as improved insulin sensitivity and reduced liver fat accumulation. Overall, the triple-agonist mechanism of retatrutide aims to address multiple facets of energy balance and glucose metabolism concurrently for a more robust therapeutic effect.
Chemical Makeup
Molecular Weight: 4997.7 Da (neutral mass by LC-MS; expected 4997.7, observed 4997.7)
Other Known Titles: Retatrutide; LY3437943; GGG tri-agonist (triple incretin receptor agonist)
CAS: 2381816-92-2
Research and Clinical Studies
Retatrutide and Metabolic Regulation
Retatrutide’s triple-receptor agonism translates into broad metabolic regulatory effects. In preclinical studies, retatrutide has been observed to delay gastric emptying and reduce food intake, resulting in significant weight reduction superior to that of single-hormone treatments. These effects are attributed to the combination of enhanced satiety signals (via GLP-1/GIP) and increased energy expenditure (via glucagon). Notably, the glucagon agonist component appears to raise basal metabolic rate and promote lipid oxidation. This comprehensive metabolic engagement leads to improved markers of metabolic health. Early-phase clinical data support these findings, demonstrating dose-dependent reductions in body weight and blood glucose. Researchers have also noted improvements in insulin sensitivity and other metabolic parameters under retatrutide, suggesting that the drug helps restore overall metabolic homeostasis. By targeting multiple hormonal pathways simultaneously, retatrutide may effectively tackle the intertwined issues of hyperglycemia, excess adiposity, and dysregulated energy balance that characterize metabolic disease.
Retatrutide and Weight Management
Clinical trials indicate that retatrutide produces substantial weight loss in individuals with obesity. In a 48-week Phase 2 study on adults with obesity, retatrutide treatment led to dramatic, dose-dependent reductions in body weight. Participants receiving the highest weekly dose (12 mg) experienced an average weight reduction of roughly 23–24% of their baseline body weight after 11 months of therapy, compared to minimal weight change in the placebo group. This magnitude of weight loss – averaging nearly 25% body weight (about 55–60 pounds on average) at the highest dose – exceeds what has been observed with earlier single or dual agonist peptide therapies. Even at intermediate doses (e.g. 4 mg and 8 mg), retatrutide elicited clinically meaningful weight reductions, with the majority of treated subjects achieving at least a 5% body weight loss. By 48 weeks, 83% of those on 12 mg retatrutide had lost at least 15% of their weight. These results underscore retatrutide’s potent anti-obesity effect. The weight loss was achieved alongside a safety/tolerability profile similar to other incretin-based peptides (primarily gastrointestinal side effects that were dose-related). The robust reductions in body mass observed with retatrutide highlight its promise as a powerful tool for weight management in research settings, potentially setting a new benchmark for pharmacological obesity interventions.
Retatrutide and Glycemic Control
In addition to weight loss, retatrutide has demonstrated significant benefits in glucose regulation for type 2 diabetes. A Phase 2 trial in people with type 2 diabetes showed that retatrutide therapy led to marked improvements in glycemic control over about 36 weeks. Depending on the dose, retatrutide lowered HbA1c (glycated hemoglobin) by approximately 1.3% to 2.0% from baseline, whereas placebo-treated patients saw virtually no change. In fact, at the top doses (8 mg and 12 mg weekly), retatrutide’s HbA1c reduction was greater than that achieved with a standard GLP-1 agonist (in this study, dulaglutide) given for comparison, indicating superior glucose-lowering efficacy. Alongside improved blood sugar levels, patients on retatrutide experienced substantial weight reductions in the diabetic cohort as well – the highest dose yielding nearly a 17% body weight decrease in about 8 months, far outpacing the minimal weight change on placebo. Importantly, retatrutide did not increase risk of hypoglycemia in these trials, as its mechanism is tied to glucose-dependent insulin release, and no severe hypoglycemic events were reported. Beyond lowering blood glucose and body weight, the multi-faceted action of retatrutide may confer additional metabolic benefits in diabetes. Researchers observed improvements in indicators of insulin sensitivity, and preclinical data suggest retatrutide could even ameliorate complications like diabetic kidney disease by reducing metabolic stress on organs. Overall, the evidence points to retatrutide as a promising investigational agent for achieving comprehensive glycemic control and weight management in type 2 diabetes, with the potential to improve multiple cardiometabolic risk factors simultaneously.
Retatrutide and Liver Health (NAFLD/NASH)
One especially novel aspect of retatrutide’s profile is its potential to improve liver health in the context of non-alcoholic fatty liver disease (NAFLD) and related conditions. In an obesity trial sub-study focusing on NAFLD, retatrutide produced dramatic reductions in liver fat content. After 24 weeks of treatment, liver fat (measured by MRI) dropped by over 80% relative to baseline in the higher-dose retatrutide groups, compared to virtually no change in placebo. By the end of 48 weeks, a striking finding was that approximately 9 out of 10 patients on the top two retatrutide doses saw their liver fat percentage normalize to healthy levels. In quantitative terms, mean liver fat reduction reached about 82–86% with retatrutide 8–12 mg, and the majority of treated NAFLD patients achieved a normal liver fat fraction (<5%) within months. These improvements in hepatic steatosis suggest that retatrutide may effectively resolve fatty liver disease in a high proportion of cases. Mechanistically, the glucagon receptor activation by retatrutide is believed to play a pivotal role – the liver has abundant glucagon receptors, and their stimulation promotes fatty acid oxidation and reduces hepatic fat accumulation. Researchers have noted that retatrutide’s activation of glucagon pathways appears to alleviate oxidative stress in liver mitochondria and exert anti-fibrotic effects, potentially slowing or reversing the progression of NASH (non-alcoholic steatohepatitis) in advanced fatty liver disease. These liver-specific benefits are unique among metabolic peptides: while GLP-1/GIP agonists indirectly benefit liver fat by promoting weight loss, retatrutide’s direct glucagon-mediated action in the liver may address fibrosis and inflammation more effectively. It is important to note that no medications are yet approved for NASH, so retatrutide’s early evidence of improving liver histology and function is of significant research interest. Ongoing studies are examining retatrutide’s impact on liver enzymes, fibrosis markers, and overall liver health in patients with metabolic-associated fatty liver disease. Thus far, the data indicate that retatrutide could become a valuable research tool for mitigating NAFLD/NASH, highlighting the broader systemic reach of this triple agonist beyond just weight and glucose control.
Retatrutide peptide is available for research and laboratory purposes only. Please review and adhere to our Terms and Conditions before ordering.
References
- Jastreboff AM, Kaplan LM, Frías JP, et al. Triple–hormone–receptor agonist retatrutide for obesity — a phase 2 trial. New England Journal of Medicine. 2023;389:514–526. https://www.nejm.org/doi/full/10.1056/NEJMoa2301972
- Rosenstock J, Wysham C, Frías JP, et al. Retatrutide, a GIP, GLP-1 and glucagon receptor agonist, for people with type 2 diabetes: a randomised, double-blind, placebo-controlled, phase 2 trial. The Lancet. 2023;402:529–544. https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(23)01053-X/fulltext
- Sanyal AJ, Kaplan LM, Frías JP, et al. Triple hormone receptor agonist retatrutide for metabolic dysfunction-associated steatotic liver disease: a randomized phase 2a trial. Nature Medicine. 2024;30:2037–2048. https://www.nature.com/articles/s41591-024-03018-2
- Coskun T, Urva S, Roell WC, et al. LY3437943, a novel triple glucagon, GIP, and GLP-1 receptor agonist: from discovery to clinical proof of concept. Cell Metabolism. 2022;34(9):1234–1247.e9. https://www.cell.com/cell-metabolism/fulltext/S1550-4131(22)00312-6
- Katsi V, Koutsopoulos G, Fragoulis C, Dimitriadis K, Tsioufis K. Retatrutide—A game changer in obesity pharmacotherapy. Biomolecules. 2025;15(6):796. https://www.mdpi.com/2218-273X/15/6/796
- ClinicalTrials.gov. A Study of Retatrutide (LY3437943) in Participants Who Have Obesity or Overweight (TRIUMPH-1/Program). NCT05929066. https://clinicaltrials.gov/study/NCT05929066
- ClinicalTrials.gov. A Study of Retatrutide (LY3437943) in Participants With Type 2 Diabetes Mellitus Who Have Obesity or Overweight (TRIUMPH-2). NCT05929079. https://clinicaltrials.gov/study/NCT05929079
- ClinicalTrials.gov. A Study of Retatrutide (LY3437943) in Participants With Obesity: Maintenance of Weight Loss. NCT06859268. https://clinicaltrials.gov/study/NCT06859268
- Nature Index entry for Sanyal et al. 2024 (study details & DOI). https://www.nature.com/nature-index/article/10.1038/s41591-024-03018-2
- Springer review: Retatrutide—an investigational triple agonist for obesity and diabetes (overview of safety/efficacy). European Journal of Clinical Pharmacology. 2024. https://link.springer.com/content/pdf/10.1007/s00228-024-03646-0.pdf

HIGHEST QUALITY PEPTIDES
Our products are scientifically formulated and manufactured in cGMP-compliant facilities.

FAST DELIVERY
Enjoy fast and reliable 3–5 day shipping.

Dedicated Customer Service
Our customer service team is highly knowledgeable in peptide research and its applications. We’re available 24/7 to assist you.

Tested. Verified. Trusted.
We take a laboratory-first approach to quality. Each batch is made under controlled conditions and verified by an independent lab (HPLC/MS). We only ship batches that test ≥99% purity, and we provide a full COA, including identity, methods, and chromatograms, for your review.
See the Process for Yourself
We make our peptides in our own cGMP lab. Watch the video to see how every vial is produced, tested, and handled with care.
Science Behind Our Peptides
A clear explanation of how our peptides work, their benefits, why quality matters for best results, and what you should know.
Categories
Categories
How do I know the peptides I order are exactly what the label says?
Every vial we sell comes from a lab that follows current Good Manufacturing Practices (cGMP). That means each step of production is documented and controlled. Before a batch is released, it’s tested by independent third-party labs for purity, identity, and sterility. Certificates of analysis are available so you can see the exact test results.
Are your peptides produced in a sterile and controlled environment?
Yes. The labs we work with use ISO-certified clean rooms where air quality, equipment, and handling procedures are tightly regulated. Staff are trained to pharmaceutical-grade standards. This ensures the peptides are produced in an environment that minimizes contamination risks.
What about shipping? Do the peptides remain stable in transit?
Peptides in lyophilized (freeze-dried) form are stable at room temperature for transport. Once you receive them, refrigeration is recommended to maintain long-term integrity. We package every order securely to prevent damage and ship promptly, so your vials arrive in optimal condition.
How do I know you actually make these peptides yourselves?
We operate under strict in-house protocols that follow current Good Manufacturing Practices (cGMP). That means our team oversees the entire process from sourcing raw amino acids to the final lyophilized vial. Nothing is outsourced or repackaged. This gives us full control over purity, consistency, and sterility, and it’s why we can stand behind every single vial we ship.
What should I do with the vials once they arrive?
Store them in the refrigerator, away from direct light and heat. If you need to keep them longer, some peptides can be stored frozen. Each vial comes with clear handling instructions so you know the proper conditions for stability.
What proof do you have that your peptides are legitimate?
The strongest proof is transparency. For every peptide, we can provide certificates of analysis, manufacturing documentation, and references to the published scientific research behind it. If you ever have questions, we’ll show you the data rather than ask you to take our word for it.