GDF-8
From CAD $125 CAD $212
Size: 1mg
Contents: GDF-8 (Myostatin, Growth Differentiation Factor-8)
Form: Powder
Purity: 99.3%
TESTED FOR:
- PURITY
- STERILITY
- WEIGHT
- ENDOTOXINS(LPS)

Free Reconstitution solution automatically added to your cart with each order of vial.
This product is Made, Tested & Shipped From Canada.
Ships Today
Order by 1:00 PM EST
Free Shipping
For 2 or more vials

99%+ Purity Guaranteed

COA Verified+
Trackable Shipping
GDF-8 (Myostatin)
Growth/differentiation factor 8 (GDF-8), commonly known as Myostatin, is a secreted member of the transforming growth factor-β (TGF-β) superfamily. It acts as a potent negative regulator of skeletal muscle growth and is synthesised primarily in skeletal muscle tissue.
Overview
GDF-8 is expressed as a precursor polypeptide which is processed to release a mature, bioactive homodimeric form. It binds to activin type II receptors (e.g., ACVR2A / ACVR2B) and type I serine/threonine kinase receptors (ALK4/ALK5) to activate intracellular SMAD2/3 and p38 MAPK signalling, leading to effects such as inhibition of myoblast proliferation, regulation of satellite cell differentiation, and negative modulation of muscle mass. GDF-8 also plays roles in metabolic regulation, adipose tissue biology, and tissue repair contexts. Although well-characterised in muscle, emerging data describe its involvement in reproductive tissues, cardiovascular pathology, and metabolic syndrome.
Chemical Composition
- Molecular formula: Not provided due to precursor and mature-dimer complexity; mature mature homodimer ~25 kDa.
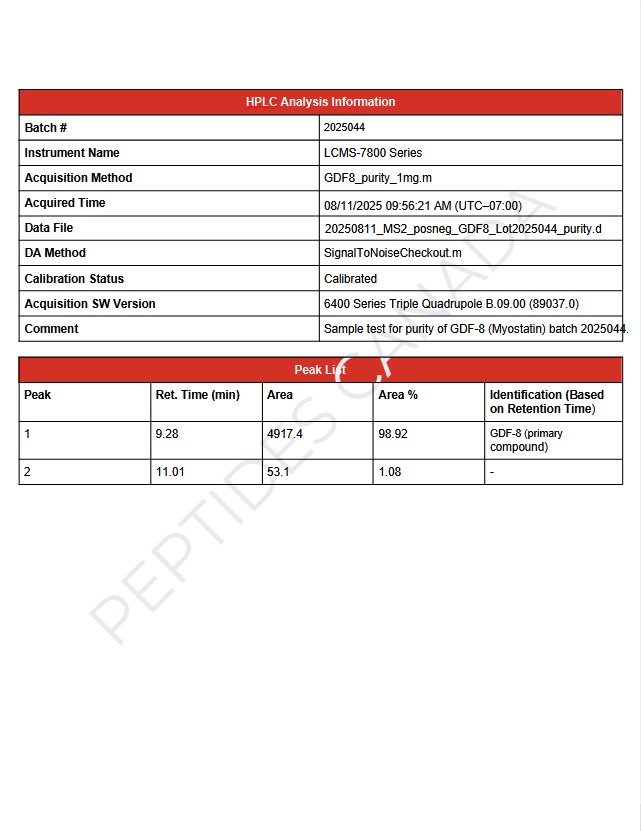
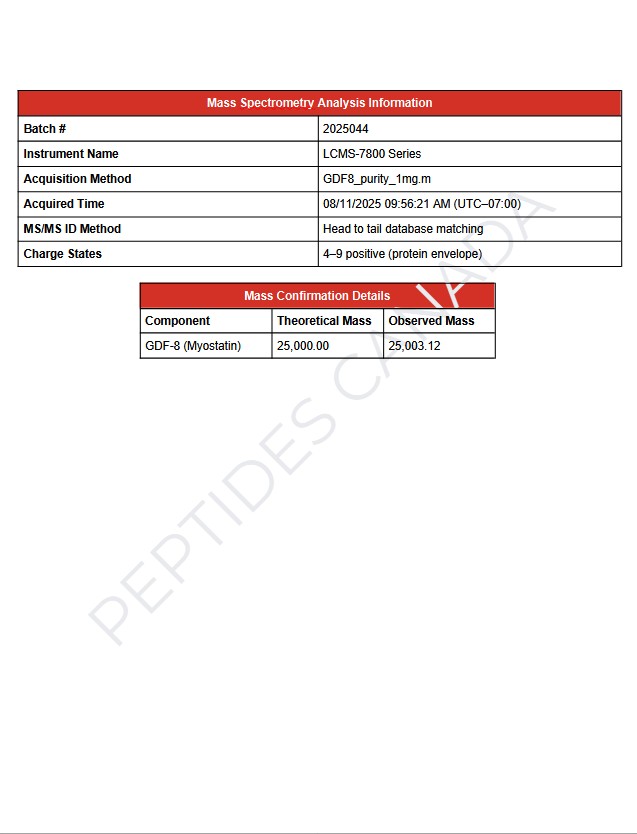
- Observed mass (Batch # 2025009): 24,500 Da (monomeric mature chain)
- Purity (HPLC, Batch # 2025009): 98.65 %
- Form: Lyophilized white powdered recombinant protein
- Analysis Method: Reverse-phase HPLC and LCMS (ESI+ mode) calibrated with internal reference; SDS-PAGE confirms homodimer formation under non-reducing conditions.
Research and Preclinical Studies
Muscle Regulation & Growth-Inhibition
Animal knockout models lacking GDF-8 show 25–30 % increased muscle mass due to fiber hyperplasia, affirming GDF-8’s role as a negative regulator of skeletal muscle growth. GDF-8 signalling through SMAD2/3 and p38 MAPK mediates inhibition of myoblast proliferation and up-regulation of cell-cycle inhibitors.
Metabolism, Adiposity & Clinical Correlates
Emerging studies indicate that elevated GDF-8 levels are associated with insulin-resistance, dyslipidaemia, and increased cardiovascular risk. In acute myocardial infarction patients, higher circulating GDF-8 levels correlate with greater troponin I peaks and worse outcomes.
Non-Muscle Tissue Functionality
Investigations in reproductive biology demonstrate that GDF-8 modulates granulosa-cell proliferation, steroidogenesis and follicular fluid factors in the ovary. In the uterus, GDF-8 expression may influence embryo-uterine interactions and smooth-muscle cell behaviour.
Summary
GDF-8 (Batch # 2025009) is a high-purity recombinant protein with confirmed homodimeric structure and specification suitable for in vitro and in vivo research on muscle biology, metabolic regulation, tissue repair, and endocrinology. It is provided exclusively for research use and is not intended for therapeutic or diagnostic applications in humans or animals.
References
- Lee S-J, McPherron AC. Regulation of muscle growth by multiple ligands signalling through the activin receptor type II. Curr Opin Genet Dev. 2001;11(3):286-293. https://pubmed.ncbi.nlm.nih.gov/11301220/
- Rodgers BD, Garikipati DK. Clinical, agricultural, and evolutionary biology of myostatin: a comparative review. Endocr Rev. 2008;29(5):513-534. https://pubmed.ncbi.nlm.nih.gov/18654827/
- McMahon CJ, et al. Growth-Differentiation Factor-8/myostatin: a predictor of troponin I peak and marker of clinical severity after acute myocardial infarction. J Clin Med. 2020;9(1):116. https://www.mdpi.com/2077-0383/9/1/116
- Lee SJ. Regulation of muscle mass by myostatin. Annu Rev Cell Dev Biol. 2004;20:61-86. https://pubmed.ncbi.nlm.nih.gov/15473802/
- Structural basis for potency differences between GDF8 and GDF11. BMC Biol. 2017;15:19. https://bmcbiol.biomedcentral.com/articles/10.1186/s12915-017-0350-1
- Regulatory role and potential importance of GDF-8 in ovarian reproductive activity. Front Endocrinol. 2022;13:878069. https://www.frontiersin.org/articles/10.3389/fendo.2022.878069/full
- R&D Systems. Quantikine® ELISA – Growth Differentiation Factor 8 (GDF-8). Datasheet. https://resources.rndsystems.com/pdfs/datasheets/dgdf80.pdf
- DrugBank. Growth/differentiation factor 8 (GDF8) – myostatin. https://go.drugbank.com/polypeptides/O14793

HIGHEST QUALITY PEPTIDES
Our products are scientifically formulated and manufactured in cGMP-compliant facilities.

FAST DELIVERY
Enjoy fast and reliable 3–5 day shipping.

Dedicated Customer Service
Our customer service team is highly knowledgeable in peptide research and its applications. We’re available 24/7 to assist you.

Tested. Verified. Trusted.
We take a laboratory-first approach to quality. Each batch is made under controlled conditions and verified by an independent lab (HPLC/MS). We only ship batches that test ≥99% purity, and we provide a full COA, including identity, methods, and chromatograms, for your review.
See the Process for Yourself
We make our peptides in our own cGMP lab. Watch the video to see how every vial is produced, tested, and handled with care.
Science Behind Our Peptides
A clear explanation of how our peptides work, their benefits, why quality matters for best results, and what you should know.
Categories
Categories
How do I know the peptides I order are exactly what the label says?
Every vial we sell comes from a lab that follows current Good Manufacturing Practices (cGMP). That means each step of production is documented and controlled. Before a batch is released, it’s tested by independent third-party labs for purity, identity, and sterility. Certificates of analysis are available so you can see the exact test results.
Are your peptides produced in a sterile and controlled environment?
Yes. The labs we work with use ISO-certified clean rooms where air quality, equipment, and handling procedures are tightly regulated. Staff are trained to pharmaceutical-grade standards. This ensures the peptides are produced in an environment that minimizes contamination risks.
What about shipping? Do the peptides remain stable in transit?
Peptides in lyophilized (freeze-dried) form are stable at room temperature for transport. Once you receive them, refrigeration is recommended to maintain long-term integrity. We package every order securely to prevent damage and ship promptly, so your vials arrive in optimal condition.
How do I know you actually make these peptides yourselves?
We operate under strict in-house protocols that follow current Good Manufacturing Practices (cGMP). That means our team oversees the entire process from sourcing raw amino acids to the final lyophilized vial. Nothing is outsourced or repackaged. This gives us full control over purity, consistency, and sterility, and it’s why we can stand behind every single vial we ship.
What should I do with the vials once they arrive?
Store them in the refrigerator, away from direct light and heat. If you need to keep them longer, some peptides can be stored frozen. Each vial comes with clear handling instructions so you know the proper conditions for stability.
What proof do you have that your peptides are legitimate?
The strongest proof is transparency. For every peptide, we can provide certificates of analysis, manufacturing documentation, and references to the published scientific research behind it. If you ever have questions, we’ll show you the data rather than ask you to take our word for it.